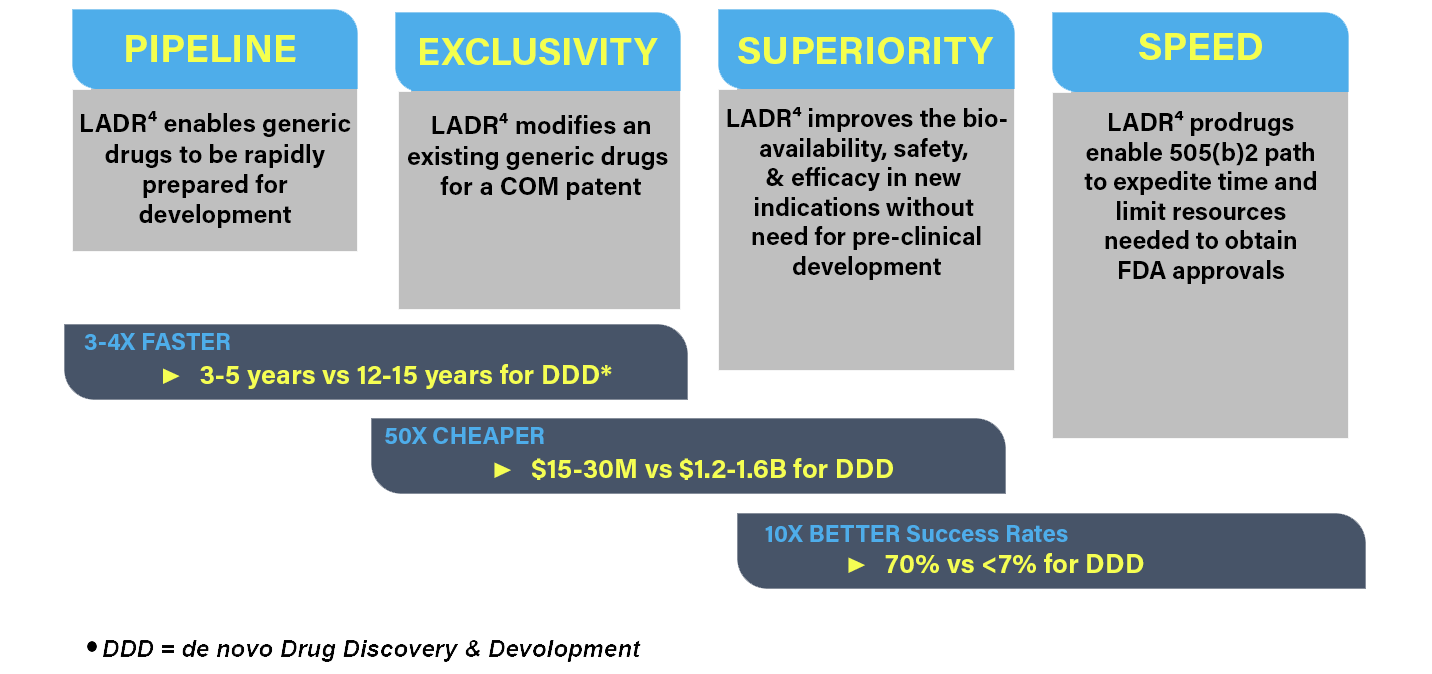Serial entrepreneur with 35+ years in drug discovery & development and co-inventor of Zetia® and Vytorin®.
LINKER-AIDED DRUG REPURPOSE, REPOSITION, REPROFILE, RESCUE (LADR4).
LADR4 is an industry first, platform technology that allows for repurposing of generic drugs by creating New Chemical Entities (NCEs) with composition of matter (COM) patent claims. These NCEs have improved physico-chemical properties and PK/PD profile, but do not trigger the need for a 505(b)1 development path. This provides a one-of-a-kind strategy that:
- Makes repurposing investable by generating a full 20 year composition of matter claim based on NCEs
- Shortens time to approval
- Lowers cost to approval
- Increases probability of success
- Provides a significant return for investors
LADR4 addresses the urgent need to develop therapies for individuals living today with rare disease, by reducing both the time and cost to obtain approval.